pharmacovigilance@lifepharmafze.com
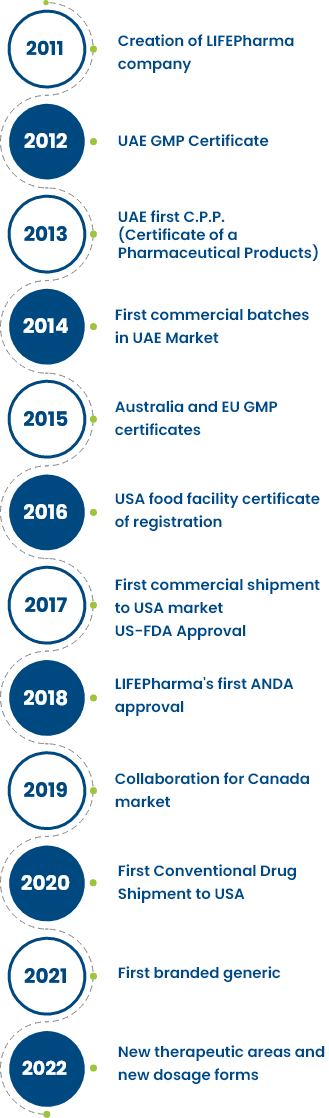
As a new step forward to expand VPS healthcare group towards pharma industry, Dr. Shamsheer Vayalil signed the establishment agreement for LIFEPharma in 2011.
Successfully achieving the manufacturing site registration from UAE MOH that is considered to be the first step in LIFEPharma journey.
In May 2013 LIFEPharma was granted the first 3 Certificate of a Pharmaceutical Products (C.P.P.). Initially, C.P.P’s were granted for 3 products and later on increased to 15 products by the end of the year 2013.
LIFEPharma started marketing its products in the UAE market from the year 2014 and remarkably achieve the market share across UAE on the same year incredibly.
LIFEPharma successfully achieved great steps to be one of the players in the global pharma business by achieving GMP accreditation from Australia and EU. Opening the doors for exportation of LIFEPharma products to these markets.
New milestone to support LIFEPharma operation globally, allowing LIFEPharma to start the business in USA by market OTC products.
LIFEPharma shipped its first consignment of Vitamin C to USA market in the year 2017.
In the same year, LIFEPharma obtained US-FDA approval for its manufacturing facility and LIFEPharma became the only US-FDA approved facility in UAE
LIFEPharma completed another milestone that helped LIFEPharma to enter USA market and support its prescence in the other markets.
LIFEPharma achieved another milestone by collaborating with Canadian pharmaceutical company to support its operation in the GCC region and new business initiation in GCC and MENA region.
First commercial shipment of conventional drugs to the USA. First to start commercialmanufacturing for Canadian market.
The launch of first branded generic of Fingolimod, Favipiravir & many other specialty molecules.
LIFEPharma included into its portfolio and
launched oncology products.
LIFEPharma launched injectable dosage forms.
LIFEPharma launched opthalmic dosage forms.